I have a ’90 Chevy pickup that is rarely driven but has been stored in my heated garage for several years. I have a new project that will force this truck outside for the winter. I live in Nebraska where it gets really cold so fresh antifreeze is going to be necessary. I was going to just buy plain, regular antifreeze but it appears that everything has changed since I last bought stuff years ago. All I see is “universal” antifreeze but I don’t even know what that means. I’ve read where GM now uses something called Dex-Cool that lasts long but others tell me to stay away from it. So what should I use?
G.L.
There have been massive changes in coolant over the last 10 years or so. It could take a small book to explain all the details so we’ll start by saying that the old school antifreeze was an ethylene glycol material that is generally colored green. This was the standard antifreeze for decades and what was used in your truck likely since it was new.
This is what you should choose as your go-to antifreeze choice.
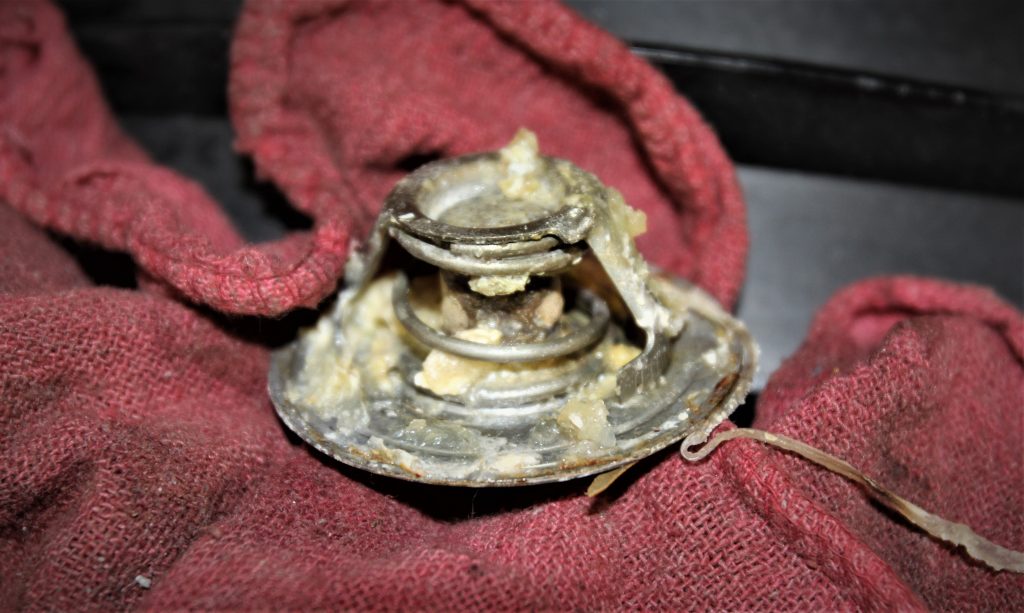
The newer materials are better and last longer but they do not always mix well. For example, the green traditional ethylene glycol is referenced as an Inorganic Acid Technology (IAT, pronounced I-at) coolant. Dex-Cool you mentioned is a completely different style of coolant, something called Organic Acid Technology or OAT (pronounced oh-at). The criticisms of Dex-Cool stem from situations when car owners mistakenly mixed their original OAT Dex-Cool with an IAT coolant. What can happen is that mixing these coolants creates a slush or gel that can be difficult to remove.
We could get into a long discussion about the different types of coolants but in the interest of not falling into a detailed chemical discussion that very few (including this author) really understands, it’s best to just stay with the recommended coolant for your vehicle.
As a 1990 truck, this would be the old standby green antifreeze but you have to be careful to be sure of what you’re buying. It should say on the label somewhere that this is an IAT or inorganic acid technology coolant. Yes there are universal coolants out there but they are often more expensive and our sources tell us that they are unsure of how they would react chemically.
Exceptions to this rule might be for a brand new engine build using a late model engine like an LS swapped into an older muscle car using a new aluminum radiator. In this case with all new components, you might consider using a coolant like the Asian formula antifreeze that is generally dyed pink or perhaps blue. This is a P-HOAT or a Phosphate Hybrid Organic Acid Technology coolant. Users of this coolant report that not only does this antifreeze last upwards of 5 years or 100,000 miles but it also does not appear to succumb to corrosion problems. Conversely, IAT or green coolant should be either changed or at least fortified with additional anti-corrosion additives.
To this end, we’ve for years used a product from Applied Chemical Specialties called No-Rosion as an anti-corrosion additive for vehicles using a brass-copper radiator. Vehicles with aluminum radiators and aluminum components can use a new formula called HyperKuhl.
Organic coolants generally contain no phosphates or silicates that can cause problems. The traditional green ethylene glycol does contain silicates that are used to prevent corrosion. Unfortunately, these silicates can also form gels if used beyond their service life. In severe cases where the vehicle has been stored with IAT coolants, these silicates can turn into something similar to a silicate gel that, if allowed to harden in the absence of water, can be extremely hard to remove from the cooling system.
The important thing to remember is that OAT coolants work better when minimally exposed to outside air that can contribute to accelerating corrosion. This means if you choose a P-HOAT coolant that the vehicle should also employ a coolant overflow reservoir that can accept overflow and then return it to the radiator when the engine cools. This reduces the chance of corrosion forming in the radiator that would be exposed to oxidation.
A final recommendation concerns the water base you should use to mix coolants. Most tap waters contain high levels of metals and other solids like magnesium and calcium that will contribute to accelerating the formation of the aforementioned gels in the cooling system. Many recommendations for using distilled water but our chemist friend Jay Ross of Applied Chemical Specialties warns that distilled water strips electrons from the water, which makes it “electron hungry” that will pull electrons from soft materials like aluminum or magnesium.
A safer selection would be soft water that has had these hard water materials removed. There is no “salt” in soft water so that should not be a concern. If soft water is not available, basic drinking water sold one gallon jugs at the grocery store is another safe alternative. As a final note, most antifreeze companies are now selling Ready to Use, Pre-Mixed gallon jugs that are a 50/50 concentration. These convenience items are priced slightly lower (about $2.00 per gallon at a local box store for example) than concentrated antifreeze. This is equivalent to paying roughly a third of the price for water.
Don’t be fooled. Buy the concentrate and pre-mix this yourself and save your money.
Hopefully this short explanation has helped with this rather confusing coolant situation. The bottom line is you can’t rely on the color (other than maybe the old original green antifreeze) and that casually mixing antifreeze chemistries may cause larger problems down the road.
Coolant Colors, Brand Names & Applications
This is a chart from Valvoline that might help point to the proper coolant for your vehicle. This is not a 100 percent accurate description but rather a recommendation of applications. It is still is a good idea to do research on recommendations for your specific vehicle because coolant is in a continual state of change.| Type | Inhibitor Technology | Zerex Coolant | Applications | Color |
|---|---|---|---|---|
| IAT (Inorganic Additive Technology) | Silicates | Zerex Original | Older Vehicles | Green |
| OAT (Organic Additive Technology) | Organic Acids | Zerex Dex-Cool | GM, Saab, VW | Orange |
| HOAT (Hybrid OAT) | Silicates & Organic Acids | Zerex G-05 | Ford, Chrysler, European | Yellow |
| HOAT (Hybrid OAT, Phosphate Free) | NAP Free | Zerex G-48 | BMW, Volvo, Tesla, Mini, Others | Turquoise |
| P-HOAT (Phosphated HOAT) | Phosphates & Organic Acids | Zerex Asian Vehicle | Toyota, Nissan, Honda, Hyundai, Kia, Others | Pink / Blue |
| Si-HOAT (Silicated HOAT) | Silicates & Organic Acids | Zerex G-40 | Mercedes, Audi, VW, Porsche, Others | Purple |
| Chart data courtesy of Valvoline | ||||


[…] A little while ago, OnAllCylinders contributor Jeff Smith penned…err…typed a very informative article on the various types of engine coolant available today. […]
I just googled you after reading an article about coolants. I recently purchased a 1980 Mercedes 450SL with 95,000 miles on it. I spent about $5000 to try and bring it up to good driving conditions, including new hoses, belts, etc. One of the items I had installed was a ac/heater servo unit valve for about $1,000. I have driven it lightly for about 200 miles, and yesterday it leaked mostly all of its green coolant. My question is what kind or brand of coolant to use, and can I safely drive it to a repair shop or should I fill it myself first before taking it in to find the problem. And would you have a guess as to what the problem might be ? I am a retired jeweler with little knowledge about cars or these kinds of toys.
Thank You very much
Hey David, thanks for reading. For starters, DO NOT drive the car without coolant. The good news is, though I’m not an expert on vintage Mercedes, I’d be willing to guess that since the leaked coolant was green to begin with, that any “green” coolant will suffice–at the very least temporarily until you can get it to a shop to fix the heater/servo unit. Click here to see pre-mixed coolant options from Summit Racing, then find one labeled GREEN. And since it’s pre-mixed, you don’t have to combine it with distilled water–just pour it directly into the radiator and drive it to your shop.
[…] primary reason for the various colors of anti-freeze is that green is frequently used to identify older antifreeze, whereas orange indicates newer, longer-lasting or extended-use antifreeze. Because ethylene glycol […]
Deionization increases corrosion, not distillation.
iam useing a new vintage air ac /heat defrost system in my 70 chevelle . i was useing evans antifreez but have been told not to use it. what kind do you beleive i should use to stop any problem . ialso useing it in a 572 big block with only alium heads.. would be thank full for a good answer as i admit i dont know everything.